The Effects of Cu@Ag Core-Shell Particles on SnBi Solder_Shenzhen Fitech
The Effects of Cu@Ag Core-Shell Particles on SnBi Solder_Shenzhen Fitech
Sn42Bi58 eutectic solder is a widely used low-temperature lead-free solder with excellent creep resistance and low melting point, which is suitable for poor heat-resistant components. In addition, it can be used in multiple reflow soldering. Sn42Bi58 eutectic solder contains a large amount of Bi, resulting in the formation of a Bi-rich layer during soldering and reliability issues such as brittleness, low conductivity, and low thermal conductivity. One of the methods to improve the reliability of Sn42Bi58 solder is to add a small amount of Ag element. Another relatively new technology is to add silver-coated copper particles to Sn42Bi58 eutectic solder (Cu@Ag).
Since Ag and Ag3Sn IMCs have excellent electrical and thermal properties, adding Sn42Bi58 solder can improve the function of the solder. However, Ag is more expensive, so Cu@Ag can be chosen instead. To verify the influence of Cu@Ag on various properties of solder joints, Li et al. conducted a series of experiments. The modified SnBi solder paste used in the experiment consists of 15% Cu@Ag core-shell particles, 75% SnBi5 eutectic particles, and 10% flux. The particle size of the Cu particles used in the experiment was 20-45μm, and the thickness of the Ag shell was 1-1.5μm.
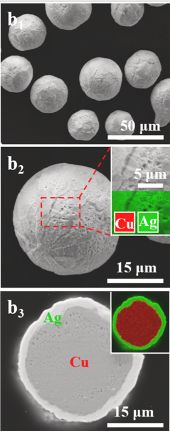
Figure 1. Enlarged images of Cu@Ag core-shell particles.
Experimental results
After adding Cu@Ag core-shell particles, an obvious Ag atomic concentration gradient appears between the shell and the solder, so Ag from the shell will continuously diffuse into the solder. During the reflow process, Ag will form Ag3Sn IMC with Sn. The emergence of Ag3Sn refines the eutectic structure to a certain extent, which is beneficial to the strength of solder joints. As the Ag shell is consumed, Cu is exposed to the solder and reacts with Sn to form Cu6Sn5.
Solder joint performance
For ordinary SnBi solder, no obvious current intensity is found in the current diagram, indicating that the overall conductivity is poor. The difference is that after Cu@Ag is added to SnBi solder, the current in both the Cu core and the Sn-rich region increases significantly when the current in the Bi-rich phase remains low. This proves that the conductivity of SnBi solder can be improved by adding Cu@Ag core-shell particles.
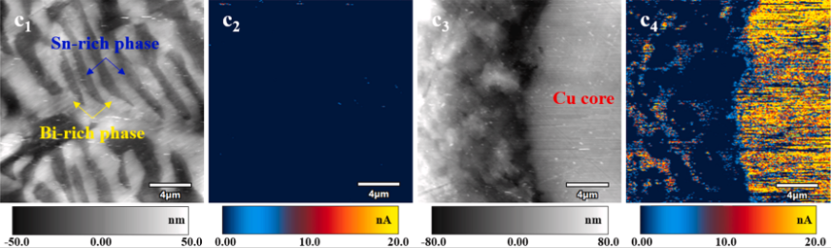
Figure 2. Solder joint current diagram. (c1-c2) Ordinary SnBi eutectic solder; (c3-c4) modified SnBi solder.
During the first reflow, the dissolution of the Ag shell does not directly affect the melting of the SnBi solder, so the melting point of the modified SnBi solder remains unchanged. The difference is that the addition of Cu@Ag core-shell particles significantly improves the thermal conductivity, electrical conductivity, and tensile strength of the modified SnBi solder. The thermal conductivity of modified SnBi solder is increased to about 41 W.m-1K-1, which is significantly higher than ordinary SnBi solder by about 90%. The electrical conductivity of modified SnBi solder is 59% higher than that of ordinary SnBi solder. The tensile strength of modified SnBi solder is increased to nearly 100MPa.
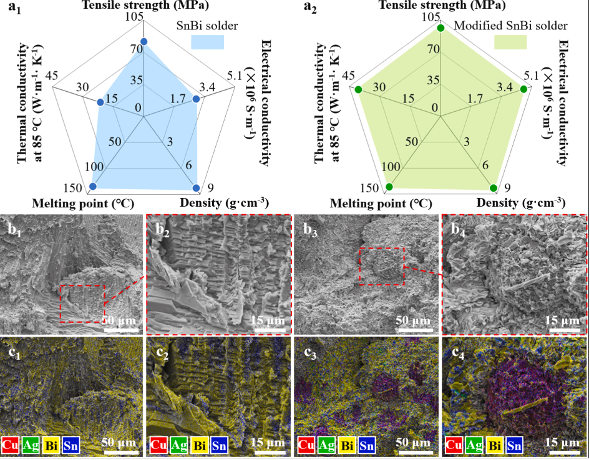
Figure 3. Comparison of performance and microstructure between ordinary SnBi eutectic solder (a1, b1, b2, c1, c2) and modified SnBi solder (a2, b3, b4, c3, c4).
Solder joint fracture mode
As shown in Figure 3, the fracture behavior of SnBi solder is determined by the interaction between the toughness of the Sn-rich phase and the brittleness of the Bi-rich phase. Cracks will tend to propagate preferentially along the brittle Bi-rich phase. Therefore, the fracture mechanism of SnBi solder is attributed to the brittle Bi-rich phase. However, after adding Cu@Ag core-shell particles, the microstructure of the solder joints showed a fine structure in the form of bumps and pits. Although the fracture surface is still dominated by the Bi-rich phase (yellow), dispersed Sn-rich phase (blue), Cu6Sn5 IMCs (purple), and Ag3Sn particles (green) appear. The solder joint fracture behavior changes from a predominantly brittle fracture mode to a brittle and ductile hybrid fracture mode.
Reference
Li, S.Q., Li, Q.H., Cao, H.J., Zheng, X.Z. & Zhang, Z.H. Significant enhancement of comprehensive properties of SnBi solder through the addition of Cu@Ag core-shell particles. Materials Science and Engineering: A, vol.881.

















 Back to list
Back to list



