Mechanism of Intermetallic Compound Formation-Shenzhen Fitech
Mechanism of Intermetallic Compound Formation
Intermetallic compounds are a class of substances formed by chemical bonding of metallic elements in specific atomic proportions.
In order to obtain a good welding result, a metallurgical reaction must take place between the solder component and the base metal component that creates a strong bond, i.e., an appropriate alloy layer (intermetallic compound, or IMC) at the interface. Therefore, in the welded joint interface, the formation of IMC or the formation of good or bad quality, the mechanical, chemical, electrical and other properties of the welded joint has a critical impact.
Solder joints serve two key purposes when joining two materials: good electrical conductivity and long-lasting mechanical joint strength.
Sn-Pb solder, for example, when the two are connected to the base metal are Cu, to achieve a durable and strong mechanical connection, it must be heated to the solder joint temperature above the melting point of the solder more than 15 ° C, the time for 2 ~ 15 s. At this time, the solder may be in the pad and components between the formation of pins between the pins of a new chemical substance, and to achieve a durable and firmly connected to the two purposes.
Obviously the formation process of intermetallic compounds is closely related to temperature and time, especially by the temperature is more obvious. Figure 1 depicts the intermetallic compounds within the welded joint in the generation process, under the action of different temperatures, the intermetallic compounds generated thickness and its impact on the strength of the welded joint.
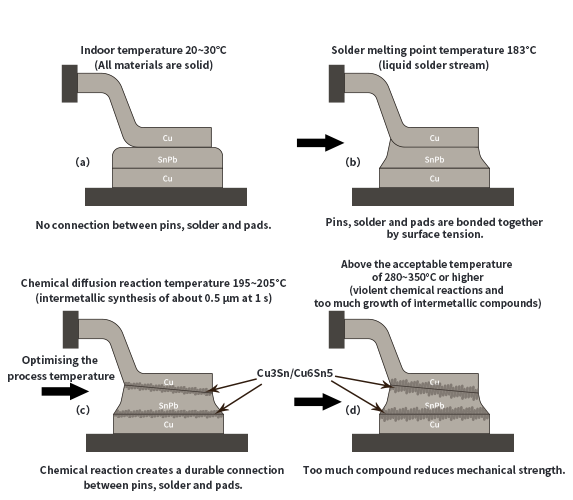
Figure 1. Metallurgical reactions at different temperature bands during the welding process
The internal structure of the solder joint at this time is shown in the figure. The intermetallic compound layer (IMC), seen under the electron microscope, consists of two substances, Cu3Sn and Cu6Sn5, as shown in Fig. 2.
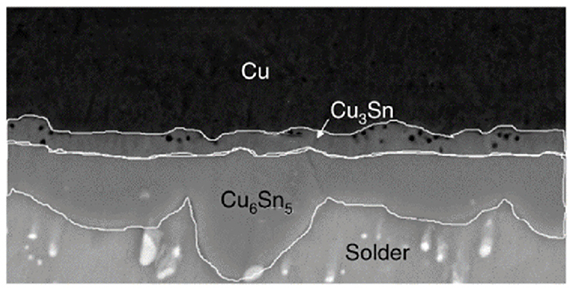
Figure 2.Composition of intermetallic compounds
The formation mechanism and morphology of Cu3Sn and Cu6Sn5 vary during the soldering process. Generally speaking, Cu6Sn5 is located on the solder side, thicker, and scalloped to grow into the liquid solder, resulting in a rough morphology at the boundary between IMC and solder; while Cu3Sn is located between the copper substrate and Cu6Sn5, thinner, and in the form of a smooth thin layer. This is because at the solid-solid interface (i.e., between the copper substrate and the solid solder), tin atoms diffuse faster to the copper substrate than to the copper atoms to the solder, resulting in the formation of tin-rich Cu6Sn5 phase first; while at the solid-liquid interface (i.e., between the copper substrate and the liquid solder), tin atoms diffuse slower to the copper substrate than to the copper atoms to the solder. At the solid-liquid interface (i.e. between the copper substrate and the liquid solder), the diffusion of tin atoms into the copper substrate is slower than the diffusion of copper atoms into the solder, resulting in the formation of the copper-rich Cu3Sn phase.
Intermetallic compounds have an important influence on the reliability of the solder interface. On the one hand, intermetallic compounds can improve the bonding strength and corrosion resistance of the interface; on the other hand, intermetallic compounds also increase the brittleness and stress concentration of the interface, and grow, transform or crack with changes in time, temperature, pressure and other factors. Therefore, when designing and fabricating lead-free tin-based soldering systems, it is necessary to consider the characteristics of intermetallic compounds such as their generation, distribution, morphology, thickness, composition, etc., and to take appropriate measures to control their quantity and quality.

















 Back to list
Back to list



