The Lead-Free Solder IMC Growth Mechanism and Impacts
The Lead-Free Solder IMC Growth Mechanism and Impacts
1. IMC at The Soldering Interface
The mainstream lead-free solders currently used are tin-silver-copper and tin-bismuth solders. When soldering with copper/nickel pads using lead-free solders, diffusion of atoms occurs at the interface of the solder and the pad due to the diffusion mechanism, producing intermetallic compounds (IMC). For example, IMC such as (Cu, Ni) 6Sn5 will grow at the interface of SAC305 solder and pad. The generation of IMC can be divided into four stages: dissolution, diffusion, solidification, and reaction processes. During the reflow process, the atoms of the solder diffuse to the surface of the substrate and form the IMC through interfacial reactions. IMC accumulates in the solder after cooling down. The diffusion process can be expressed by Fick's first law.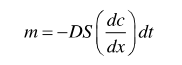
m: Diffusion flux
D: Diffusivity
S: Contact aera
dc/dx: Concentration gradient
dt: Diffusion time
2. The IMC Growh at SAC305 Micro-Bump
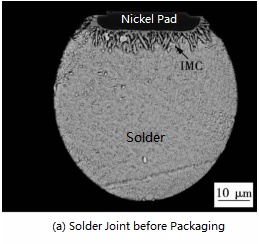
When the SAC305 solder and nickel pads form a micro-bump, an IMC layer appears between the pad and the solder. It can be seen from the cross-sectional backscattered electron image (BSE) (Fig. 1) that the IMC of the thin black layer on the side of the nickel pad is Ni3P. The needle-like IMC is (Ni, Cu)3Sn4, and the IMC of the smaller white particles is Ag3Sn. (Cu, Ni)6Sn5 is initially formed at the interface. However, (Cu, Ni)6Sn5 begins to dissolve as the Cu content is depleted. Cu atoms precipitate into Ni3Sn4 and replace Ni to form (Ni, Cu)3Sn4.
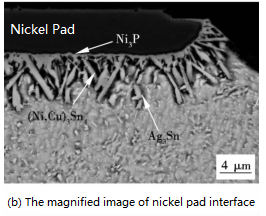
The Cu content in the solder is the main factor affecting the IMC of the Ni/SnAgCu interface (Tian et al., 2013). When the Cu content is 0.4-0.6%, the interface forms (Cu, Ni)6Sn5 and (Ni, Cu)3Sn4, while (Ni, Cu)3Sn4 is generated below this range. (Cu, Ni)6Sn5 is formed if the Cu content is higher than 0.6%. In addition, Ni atoms can reduce the solubility of Cu atoms in tin solder and affect the growth of (Cu, Ni)6Sn5 (Tian et.al., 2013).
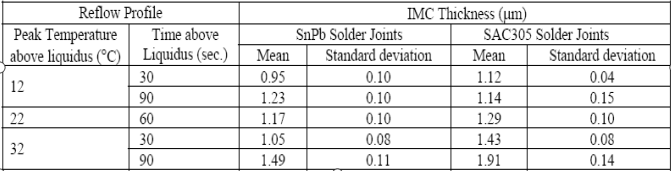
The diffusion process continuously happens between atoms. The layer in the IMC becomes thicker with increasing temperature and time. As can be seen from the table below, higher peak reflow temperature and longer time speed up the growth of the IMC.
Table 1: The impacts of reflow temperature and time on IMC thickness.
3. The Impacts of IMC
A thin and evenly distributed IMC can enhance the solder joints. However, the IMC is brittle, and large pieces of IMC will appear when processing thermal aging, which will increase the internal stress and brittleness of the solder joints. If applying an external force, the solder joints are likely to crack and fail. After the SAC305 micro-bumps and copper pads are soldered, long-term thermal aging will induce Cu3Sn growth on the Cu pad side and weaken the strength of the solder joints.
4. Reference
Tian, Y., Wu, Y.P., An, B., & Long, D.F. (2013), "The Formation and Growth of Intermetallic Compound at the Interface of Fine-Pitch Flip-Chip when Interconnecting”, Transactions of the China Welding Institution, 34(10).

















 Back to list
Back to list



