The Introduction of Eutectic and Eutectic Solder Pastes
The Introduction of Eutectic and Eutectic Solder Pastes
1. Eutectic and Phase Diagram
Unlike the phase transitions of pure substances, alloys have an intermediate phase. Different proportions of solid and liquid mixtures appear at different temperatures. The phase transition temperature depends on the substance ratio and the degree of mixing. A eutectic can be defined as a mixture with a particular component ratio that has a melting/solidification temperature lower than its individual components or mixtures with different component ratios. A eutectic phase diagram is a tool to help understand the relationship between different alloy ratios and melting point temperatures. At present, binary and ternary phase diagrams are commonly used. The addition of elements increases the difficulties for phase determination.
2. Eutectic Solder Paste Introduction
Eutectic solder pastes have been commonly used in semiconductor packaging. The alloy ratios of eutectic solder pastes are confirmed by phase diagrams. In order to meet different soldering requirements, the melting point of the solder paste will vary accordingly. Some components require the use of low-temperature solders, and eutectic solder pastes are able to reflow soldering with lower temperatures. Eutectic solder pastes are bright, while non-eutectic solders tend to be darker in color.
For example, the figure below is a binary eutectic phase diagram of a tin-lead solder alloy. The temperatures along the liquidus are the melting temperatures of the solder pastes with different metal ratios. When the tin composition is 100%, the metal melting point is 232°C, while the metal melting point for pure lead is 327°C. It can be seen from the figure that the solidus and the liquidus coincide at 183°C, where the liquidus and solidus temperatures are the smallest. The composition ratio of tin at the eutectic point is 63%wt, and the lead is 37%wt. If the proportion of tin decreases, the liquidus temperature will increase, and vice versa. In addition, changing the proportion of tin from the eutectic point leads to the appearance of solid solutions, such as αPb+ liquid and βSn+ liquid.
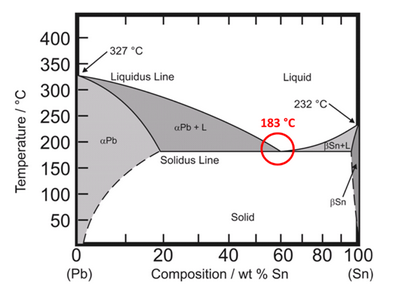
Figure 1: Sn-Pb phase diagram
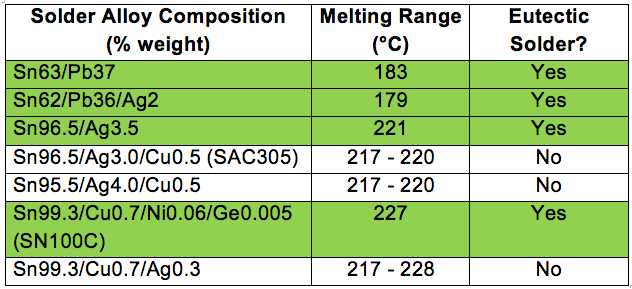
As leaded solders are restricted, lead-free solders are becoming mainstream. The table below is a selection of common lead-free solder alloys, some of which are eutectic types.
Table 1: Common lead-free solder alloys
3. The Features of Eutectic Solder Paste
Eutectic solder pastes are famous for their lower alloy melting points. The low melting point allows the solders to melt and solidify faster during reflow, accelerating the packaging process. Non-eutectic solders produce solid-liquid mixtures that are likely to cause solder joint cracking, while eutectic can reduce the solder joint cracking. In addition, the solid-liquid temperature ranges of the eutectic are small, and the liquid flow resistance is small. Hence, the eutectic alloys have better fluidity.
Shenzhen Fitech provides high-quality eutectic solder pastes for microelectronics and semiconductor packaging. Welcome to consult.

















 Back to list
Back to list



