Mechanism of Oxide Film Removal by Active Substances of Solder Paste Flux
Before soldering, there is often an oxide film on the surface of the solder alloy powder and the surface of the metal pads. These oxide films must be fully removed to ensure smooth soldering. The oxide removal is completed due to the activity of the solder paste flux. What is the mechanism of the active substances in the solder paste flux to remove the oxide film?
In the early days, the ability of solder paste flux to remove oxide film was mostly explained by chemical dissolution and film removal mechanisms such as rosin or electrochemical principle. Later, according to the characteristics of the raw materials used in the solder paste flux, the principles of complex chemistry and polymer chemistry were used to discuss the mechanism of the solder paste flux, and the chemistry of the active substances in the solder paste flux was explained. The flux activity is mainly manifested in its oxophilicity. Before reaching the soldering temperature, the molten solder of the solder paste should be fully reduced or replaced with the oxides on the surface of the base metals to form new metal salt compounds or coordination compounds of metal ions, such as CuCI2, copper rosinate, Copper stearate, copper bromide, etc. Some of the compounds or coordination compounds can be dissolved in rosin to form flux residues, and some have good flux performance that can enhance the wetting ability of solder paste flux at the soldering temperature. For example, copper stearate has a better fluxing effect than stearic acid and can increase the flooding area for the solder on the Cu surface.
Copper stearate is light green and thermally decomposes at 230 °C. During the decomposition process, [H]+ in the hydroxyl group is obtained from the polymer system, which is repolymerized into stearic acid, and active copper is precipitated. The generated stearic acid can interact with Cu oxides again and again. Its reaction equation is as follows.
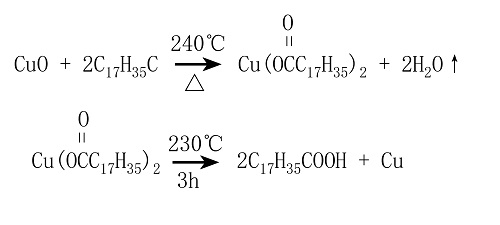
The above-mentioned reaction is carried out over and over again. The quantity of copper stearate participating in the reaction is constant, and the reaction process is an exothermic reaction. Therefore, this process is essentially a catalytic reaction. Because this reaction is carried out on the contact surface of the molten solder paste, the solder paste flux, and the base metals, it is also a heterogeneous complex catalytic reaction. Because of the existence of this reaction process, the solder removal process can be carried out in an all-round way.
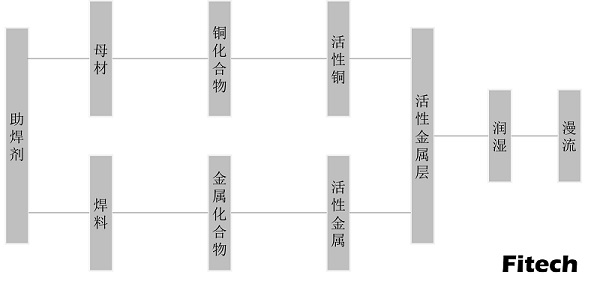
Figure 1. Mechanism of solder flux.
The temperature at which the cleaning reaction of the active material occurs is called the activation temperature. Since the activation temperature is very close to the decomposition temperature of the active material, the active material decomposes and evaporates after cleaning the metal surface. The rosin resin, epoxy resin, etc., cover the cleaned metal to prevent surface oxidation.

Taking diethylamine hydrochloride as an example, the chemical reaction of its cleaning process is as follows.
-End-
Fitech is a comprehensive solder paste supplier integrating production, sales, research, and service of solder paste, epoxy solder paste, and ultra-fine alloy solder powder, which has a complete product line from alloy solder powder to application products. The products cover ultra-fine lead-free solder paste, high/low-temperature solder paste, die bond solder paste, gold-tin solder paste, etc. Fitech is an electronic-grade packaging material manufacturer that can manufacture T2-T10 ultra-fine alloy solder powder and is the unit in the formulation of solder powder standards by the Ministry of Industry and Information Technology. Welcome to inquire about and customize products.
*Disclaimer: Except for "reprinted" articles, the copyright of the original content published on this site belongs to Shenzhen Fitech. Without consent and authorization, it may not be reproduced, reproduced, quoted, changed, or published. This article is originally created by the author, and the content of the article is the author's personal opinion. "Reprint" is only to convey a different point of view and does not mean approval or support for the point of view. If there is any infringement, please contact us, and we will delete it!

















 Back to list
Back to list



