Pad surface treatment in microelectronics and semiconductor packaging-Shenzhen Fitech

Pad surface treatment in microelectronics and semiconductor packaging
In microelectronics and semiconductor packaging, the most basic purpose of pad surface treatment is to ensure good solderability and conductivity of the pads. Since copper tends to exist as oxide in air and is unlikely to remain as pristine copper for long, other treatments are required for copper. Although in the subsequent assembly, strong flux can be used to remove most of the copper oxide, but the strong flux itself is not easy to remove, so the industry generally does not use strong flux. Common PCB surface treatment processes are OSP, HASL, IAg, ISn, chemical nickel gold, chemical nickel palladium gold, chemical thin nickel palladium gold, electroplated nickel gold and electroplated gold, the following will be introduced one by one.
OSP
OSP is a layer of organic solder film over the copper foil of the PCB pad, which melts/cracks away when soldering, and the solder reacts with the copper foil of the pad to produce the scallop-shaped Cu6Sn5 and nano-thick Cu3Sn metal compounds.
The leaded spray tin plate is a tin-lead eutectic alloy of 6337 impregnated on the surface of the PCB copper foil. The lead does not participate in the formation of IMC, tin reacts with copper pads to form a scallop-shaped Cu6Sn5; and nano-thickness of Cu3Sn, the solder replenished during the soldering process with the spray layer to maintain the reaction between tin and copper, forming a tin-copper intermetallic compound: lead-free spray solder is a PCB solder pad copper foil dipped in tin-copper solder, tin reacts with copper pads to form a scallop-shaped Cu6Sn5; and nano-thickness of Cu3Sn. Cu3Sn, trace amounts of copper in the solder melted in the solder, in principle, do not participate in the formation of IMC.
IAg
Chemical precipitation silver plate is a chemical displacement reaction on the PCB pad copper foil covered with a layer of silver, silver quickly melts into the liquid solder during the soldering process, the tin in the solder reacts with the pad copper foil to form scallop-like Cu6Sn5 and nanometer thickness Cu3sn, the silver layer does not participate in the generation of IC.
ISn
Chemically immersed tin plates are covered with a layer of pure tin through a chemical substitution reaction on the copper foil of the PCB solder pad.The pure tin layer and the PCB copper foil form a tin-copper intermetallic compound, the scalloped Cu6Sn5; and the nano-thick Cu3Sn, which is continuously replenished with tin in the liquid solder during the soldering process for effective IMC generation.The liquid solder is continuously replenished during the soldering process to provide effective IMC production.
Electroless Nickel Gold
Electroless nickel-gold (also called electroless nickel-impregnated gold) is a layer of nickel 3~5 μm thick on the PCB copper foil by chemical reduction reaction, and then a layer of gold 50~150 nm by chemical replacement reaction (some manufacturers have reduced the thickness of gold layer to 25~100 nm in recent years), the nano-thick gold layer quickly melts and diffuses into the liquid solder during the soldering process, exposing the nickel layer and the solder to produce Small grass-like Ni3Sn4, or Ni3Sn2.
Electroless Nickel-Palladium
Electroless Ni-Pd-Au is a 3~5 μm layer of nickel deposited on the PCB copper foil, a 30~70 nm layer of palladium on top of the nickel, and another 30~50 nm layer of gold on top of the palladium. During the soldering process, both the gold and palladium layers will quickly melt into the liquid solder, exposing the underlying nickel and solder to form a small grass-like tin-nickel intermetallic compound Ni3Sn4 or Ni3Sn2.
Chemical thin nickel palladium gold
Chemical nickel palladium gold is to sink a 3~5 μm nickel layer on the PCB copper foil, make a layer of 30~70 nm palladium on the nickel, and then palladium on it Make a layer of 30~50 nm gold. During the soldering process, both the gold and palladium layers are rapidly melted into the liquid solder, exposing the bottom layer Nickel forms a grassy tin-nickel intermetallic compound Ni3Sn4 or Ni3Sn2 with solder.
Electroplated Nickel Gold
The thick gold layer melts into the liquid solder when soldering and may generate brittle Ausn4, which affects the reliability of the solder joints, therefore, the industry generally does not use electroplated Ni-Au as the soldering surface. If you must use electroplated thick nickel gold as the soldering surface, generally require soldering before doing the gold enamel tin processing. The gold layer melts into the liquid solder, and the bottom nickel layer comes into contact with the liquid solder to produce small grass-like Ni3Sn4 or Ni3Sn2.
Electroplating
Gold plating is the direct electroplating of gold on PCB copper foil, because copper and gold fuse with each other, the gold plating layer must have a certain thickness to ensure that the gold layer is not consumed by the underlying copper melting and affect the performance. Generally, direct electroplating of copper foil is not used as a soldering interface.
Table 1. Different alloy parameters at the tin-copper interface
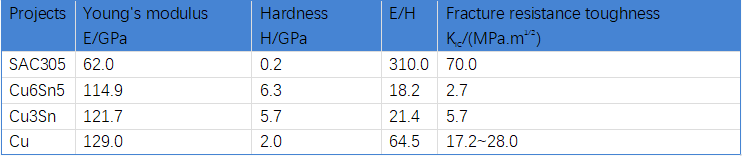
From the above analysis, it is clear that there are only two main states of the solder joint interfacial alloy layer (IMC layer): tin-copper intermetallic compound and tin-nickel intermetallic compound. The solder joint consists of three parts: the solder, the interfacial alloy layer (IMC layer), and the PCB copper foil. The mechanical strength of copper and solder in these three is greater than the mechanical strength of the interfacial alloy layer, because the interfacial alloy layer is "brittle". Within metallurgy there is no indicator to assess the "brittleness" of this material, so the plasticity and fracture toughness of the material is used to measure. The plasticity of the material refers to the ability of the material to deform within the elastic range, which is generally proportional to the ratio of Young's modulus E to hardness H. The fracture toughness refers to the ability of the material to resist crack expansion when subjected to stress, and the lower the value, the more likely the material is to crack when subjected to stress. Take the tin-copper interface alloy as an example, as shown in Table 1, the solder E/H value and the copper foil itself E/H value are much larger than the IMC layer ratio; fracture resistance toughness index shows that the solder and copper foil value is as much as several times the IMC layer value. This is the reason why the cracking surface of the solder joint always appears in the IMC layer when it is subjected to mechanical or thermal stresses. Therefore, the mechanical strength of the solder joint does not depend on the type of solder, but in most cases is determined by the structure of the IMC layer and the shape and thickness of the IMC layer. It is not that the choice of alloy composition is not important, but because the alloy composition is different, the CTE of the solder joint is different, the ductility of the solder joint is different, the resulting IMC morphology thickness is different, and the effect of the solder joint when subjected to shear and tensile forces is different. For example, lead eutectic solder, IMC formation is the result of the reaction between tin and copper, but with IMC growth and aging, the grains become coarse, lead as filler can fill the voids left by the grain growth, which to a certain extent delayed the failure of the solder joint aging. Lead-free solder SAC series does not have this process, so the reliability of lead-free solder is lower than the traditional leaded solder joints, but the probability of failure of the solder joints due to purely mechanical strength differences in the composition of the solder is extremely low. However, the various alloy properties described above are still one of the factors to be considered when selecting the alloy composition.

















 Back to list
Back to list



