Mechanism of P segregation at the weld interface of ENIG Ni(P) coatings_Shenzhen Fitech
Mechanism of P segregation at the weld interface of ENIG Ni(P) coatings_Shenzhen Fitech
P segregation is a phenomenon that occurs when metallurgical reactions between Ni and Sn in the solder lead to an enrichment of P in the Ni(P) layer near the weld interface when reflow soldering with ENIG Ni(P) layers.P segregation reduces the strength and reliability of the weld interface, and increases the brittleness of the weld joint and the risk of cracking.
When SnPb is used to weld on the Ni(P) layer, when the molten solder and the Ni(P) layer are in contact, the Ni3Sn4 intermetallic compound generated by the metallurgical reaction between the Ni in the Ni(P) layer and the Sn in the solder consumes the Ni in the area close to the solder layer, resulting in the emergence of a P-rich layer in the area, which leads to P segregation, as shown in Figure 1.
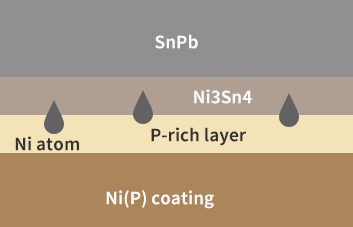

Figure 1. P segregation occurred when SnPb solder was soldered with Ni(P) coating.
When using lead-free solder SAC solder on Ni (P) layer, the situation is basically similar to leaded solder, the difference is that the intermetallic compounds generated at this time for Sn, Cu, Ni ternary alloy, as shown in Figure 2.

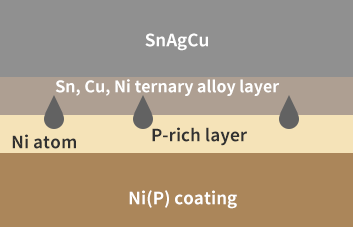
Figure 2. SAC solder and Ni (P) layer when welding the P segregation occurs
From the above two cases, it can be seen that the mechanism of P segregation is mainly due to the dissolution of Ni and the enrichment of P. The dissolution of Ni is due to the metallurgical reaction between Ni and Sn, while the enrichment of P is due to the diffusion of P and the depletion of Ni. P segregation will lead to a reduction in the strength and reliability of the weld interface because P is a brittle element, which makes the structure of the intermetallic compound unstable and fragile. P is a brittle element, which makes the structure of intermetallic compounds unstable and fragile, thus increasing the brittleness of welded joints and the risk of cracking.
The main factors affecting P segregation include the composition of the solder, the thickness of the Ni(P) layer, and the soldering temperature and time.
1. The composition of the solder: the composition of the solder affects the rate and type of metallurgical reaction between Ni and Sn, which in turn affects the degree of Ni dissolution and P enrichment. Generally speaking, the higher the content of Sn, the more obvious the dissolution of Ni and the enrichment of P, because Sn will promote the diffusion and consumption of Ni.
On the other hand, the addition of a certain amount of Cu or Ag can effectively inhibit P polarisation because they can form stable intermetallic compounds that hinder the diffusion of Ni. Figure 3 represents the effect of solder alloy composition on the thickness of P-rich layer and Ni-Sn compound layer.
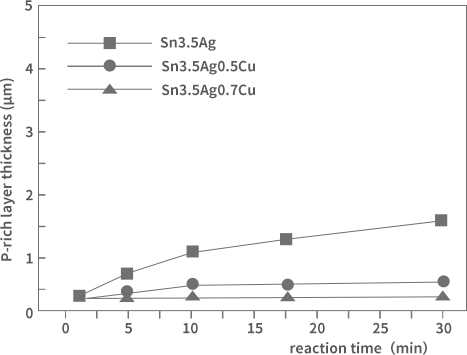
Fig. 3. Effect of SAC composition on the variation of the thickness of P-rich layer
As can be seen from the figure, the growth of the P-rich layer is more significant for the Sn3.5Ag binary alloy. As for the ternary alloy with added Cu, there is only a thickness of a few hundred nm even after 30 min of reaction, and Cu constitutes a blocking layer for Ni diffusion. Therefore, the different content of Cu in the solder has a greater impact on the formation of the interface layer.
2. Ni (P) layer thickness: Ni (P) layer thickness will affect the degree of Ni dissolution and P enrichment, thus affecting the severity of P segregation. Generally speaking, the greater the thickness of Ni(P) layer, the higher the content of P, the more serious the degree of P segregation, because the diffusion of P and the consumption of Ni the greater the space. When the thickness of Ni (P) layer is less than 3μm, the effect of P segregation is negligible.
3. Welding temperature and time: welding temperature and time will affect the rate and type of metallurgical reaction between Ni and Sn, thus affecting the degree of Ni dissolution and P enrichment. Generally speaking, the higher the welding temperature and time, the more obvious the dissolution of Ni and P enrichment.

















 Back to list
Back to list



