Lead-Free Silver-Free Solder Paste Solution for Silver Migration
Lead-Free Silver-Free Solder Paste Solution for Silver Migration
Solder Paste and Ultra-Fine Solder Manufacturer-Shenzhen Fitech is a comprehensive solder paste supplier integrating production, sales, research, and service of solder paste, epoxy solder paste, and solder powder. Fitech is the leading unit for the formulation of solder powder standards of the Ministry of Industry and Information Technology. Fitech's products include ultra-fine lead-free printing solder paste, ultra-fine lead-free dispensing solder paste, ultra-fine lead-free jetting solder paste, ultra-fine lead-free pin transfer solder paste, no-clean solder paste, water-soluble solder paste, high-temperature solder paste, medium-temperature solder paste, low-temperature solder paste, etc. Fitech can manufacture electronic-grade packaging solder powders with particle sizes from T2-T10.

Today, let's talk about a phenomenon in semiconductor devices called silver migration. Silver migration affects reliability (silver coating, silver soldering, and metallic silver are electrodes to reduce the insulation resistance decreases, eventually forming a short circuit and failure). Metal migration occurs not only with silver but also with other metal elements (lead, copper, tin, gold, etc.); Besides, metal migration occurs in semiconductor devices and other places where metal elements are susceptible to migration.
Silver is widely used in electrical connections and electronics because of excellent electrical conductivity, heat transfer, solderability, and low contact resistance. However, silver is the metal with the highest mobility of all the migrated metals (1000 times that of copper). Therefore, the migration phenomenon is known as silver migration.
In 1950, silver migration was discovered by using two silver rods as electrodes, making them 12.5mm apart. Sandwiching the filter paper between electrodes and applying a voltage of 45V at a relative humidity of 98% for 36 hours were the experimental conditions at that time. The final results show that silver grew from the cathode to the anode to form dendrites. Silver oxide (Ag2o) colloids diffused from the anode to the cathode.
Definition of silver migration: Under appropriate conditions, silver moves from an initial location to an extreme region to redeposit.
Silver migration can be divided into ion migration and electron migration.
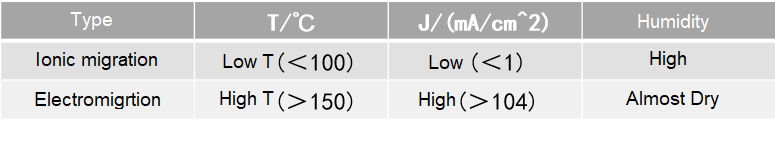
The following reaction formulas explain anion migration:
①The metallic silver is ionized due to the potential difference and the adsorbed electrolyte (water in most cases) from the environment.
Ag→Ag+, H2O→H++OH-
②AgOH decomposes to form Ag2O. Ag2O is formed at the anode, which is dispersed in colloidal form.
2AgOH→Ag2O+H2O
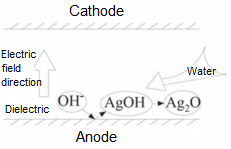
③Ag+ and OH- produce AgOH precipitates at anode.
Ag++OH-→AgOH
④Ag2O reacts with water to form anions that transfer to cathode and precipitate, showing a dendritic shape.
Ag2O+H2O <-> 2AgoH<->2Ag++OH-
We can know that the necessary conditions for anion migration are electrolytes (usually water), potential differences, and migration paths.

We can take some protective measures based on the necessary conditions:
① Adopting air tightness and baking before sealing to avoid introducing electrolytes;
② Reasonable wiring to ensure the sufficient spacing and proper operations of dispensing, patching, welding, etc., to reduce potential difference;
③ Coating the surface with a polymer film layer to block the silver migration path.
Source: 功率半导体那些事儿, retrieved by Fitech


















 Back to list
Back to list



