What Is Black Pad?
What Is Black Pad?
Copper is commonly used as the pad material in the packaging process. To avoid copper oxidation and improve the solderability of copper pads, various surface treatment methods are used to coat metal layers on copper pads. Common surface treatment processes include OSP, electroless nickel immersion gold (ENIG), etc. The ENIG is performed by electroless nickel plating on the copper pads. The nickel-plated pads are then immersed in gold water to achieve gold plating through the displacement reaction. The gold layer can protect the nickel layer from oxidation.
The ENIG process has the risk of the black pad. The reason causing the black pad is that the atomic radius of nickel is smaller than that of gold, resulting in rough surface grains after gold plating. The gold plating solution will penetrate the nickel layer where an interfacial reaction occurs and corrode the nickel layer. The corroded nickel atoms will continue to oxidize and generate nickel oxide with poor solderability. Because the color of nickel oxide is black and dark, this phenomenon is called black pad. In addition, when the gold layer is generated, the gold ions in the gold plating solution absorb electrons from the nickel layer, and nickel ions are released (Figure 1). Since the nickel layer contain a certain proportion of phosphorus (7-10%), a phosphorus-rich layer will form on the surface of the nickel layer with the release of nickel ions. The connection between the phosphorus-rich layer and the copper pad is weak. Consequently, solder joints are easily affected by an external force after soldering, which leads to solder joint failure.
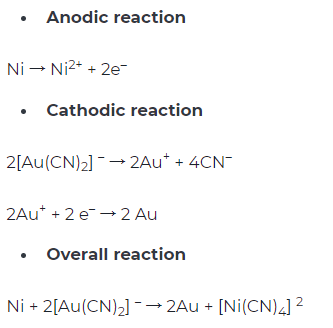
Figure 1: The mechanism of displacement reaction to plate a gold layer.
The gold layer produced by the ENIG process only has a thickness of 0.05-0.1μm. If the processing time and temperature are not controlled properly during the gold plating process, it is likely to cause uneven gold layer thickness, leading to a corroded pad. If the gold layer is too thick, the possibility of the black pad effect will increase. An unreasonably thin gold layer causes the nickel layer susceptible to corrosion by the gold plating solution. As shown in Figure 2, the black cracks in the nickel layer indicate that the nickel layer is severely corroded, while the outermost gold plating layer does not appear observable color change. As a result, it is hard to judge whether a black pad occurs from the appearance. The gold layer dissolves rapidly into the solder while heated. However, the exposed nickel oxide cannot form intermetallic compounds with tin, leading to poor connection. The black pad will degrade the solderability of the pads. Besides, it increases the solder joint brittleness and reduces fatigue resistance, leading to component failure.

Figure 2: The SEM image of nickel layer.
Although it is difficult to detect the black pad through appearance, there are some measures to prevent the black pad occurrence. For example, routine maintenance of the bath can be performed, and the temperature can be controlled to ensure proper coating. Monitoring the pH of the bath can also be implemented to ensure that the corrosiveness is within acceptable limits.

















 Back to list
Back to list



